Heat
advertisement
Heat
From what height should fall a mass of water so that its final energy, converted into heat, increase the
temperature of this mass of 1 °C? Assume that there is no energy loss in the system.
Data: 1 cal = 4.18 J, g = 9.8 m/s2, c = 1 cal/g°C.
Data: 1 cal = 4.18 J, g = 9.8 m/s2, c = 1 cal/g°C.
A body made of 250 g of brass is heated from 0 °C to 100 °C, for this 2300 cal were used. Calculate:
a) The specific heat of the brass;
b) The heat capacity of the body;
c) If the body in the final situation loses 1000 cal, what will be its temperature?
a) The specific heat of the brass;
b) The heat capacity of the body;
c) If the body in the final situation loses 1000 cal, what will be its temperature?
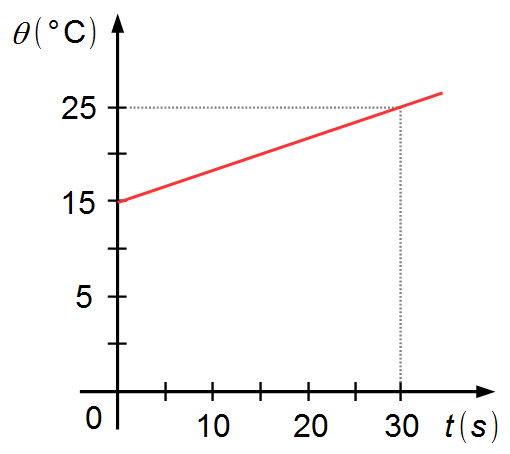
A body of mass 200 g is heated for 30 seconds by an energy source that provides a power of 210 W
at a constant rate. Given the graph of temperature as a function of time, determine the heat capacity
of the body, knowing that 1 cal = 4.2 J.
Determine the heat required to convert 100 g of ice at −10 °C in 100 g of steam at 100 °C. Also,
plot a temperature graph as a function of the quantity of heat of the phase changes. Data:
specific heat of ice: ci = 0.5 cal/g°C;
latent heat of fusion: LF = 80 cal/g;
specific heat of water: cw = 1.0 cal/g°C;
latent heat of vaporization: Lv = 540 cal/g.
specific heat of ice: ci = 0.5 cal/g°C;
latent heat of fusion: LF = 80 cal/g;
specific heat of water: cw = 1.0 cal/g°C;
latent heat of vaporization: Lv = 540 cal/g.
A body has a mass of 500 grams and specific heat of 0.4 cal/g°C. Determine:
a) The quantity of heat that the body should receive to ensure that its temperature varies from 5 °C to 35 °C;
b) The quantity of heat that the body must give so its temperature decreases from 15 °C.
a) The quantity of heat that the body should receive to ensure that its temperature varies from 5 °C to 35 °C;
b) The quantity of heat that the body must give so its temperature decreases from 15 °C.
A blender has a power of 84 W and is used to stir 100 g of water for 30 seconds. It is assumed that all the
work is transferred as heat to the water inside the blender and that there are no other energy transfers.
Calculate the heating of the mass of water. Given: 1 cal = 4.2 J.
A blender has a power of 90 W and is used to stir 200 g of water for 1 minute. The blender loses 10% of its
energy through dissipation, the other 90% of the energy is the work transferred as heat to the water inside
the blender. Calculate the heating of the mass of water. Given: 1 cal = 4.2 J.
advertisement

Fisicaexe - Physics Solved Problems by Elcio Brandani Mondadori is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License .