Solved Problem on Heat
advertisement
A blender has a power of 84 W and is used to stir 100 g of water for 30 seconds. It is assumed that all the work is transferred as heat to the water inside the blender and that there are no other energy transfers. Calculate the heating of the mass of water. Given: 1 cal = 4.2 J.
Problem data:
- Blender power: \( {\mathscr P} = 84 W \);
- Mass of water: m = 100 g;
- Time interval: Δt = 30 s;
- Specific heat of water: c = 1 cal/g°C;
- Mechanical equivalent of heat: 1 cal = 4.2 J.
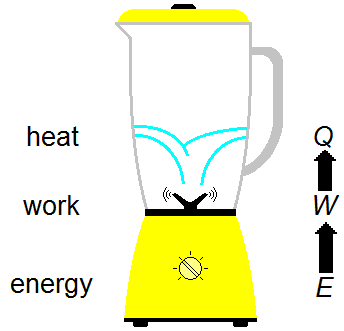
All the energy E produced by the electric motor is used in work W to produce heat Q,
which heats the water (Figure 1).
\[
\begin{gather}
E=W=Q
\end{gather}
\]
Solution:
The power delivered by the blender during the running time is given by
\[
\begin{gather}
\bbox[#99CCFF,10px]
{{\mathscr P}=\frac{E}{\Delta t}}
\end{gather}
\]
\[
\begin{gather}
E=\mathscr P\Delta t \\[5pt]
E=84\times 30 \\[5pt]
E=2520\;\mathrm J
\end{gather}
\]
Converting the calculated energy in joules (J) to calories (cal) used in the problem
\[
\begin{gather}
E=2520\;\mathrm{\cancel J}\times\frac{1\;\mathrm{cal}}{4.2\;\mathrm{\cancel J}}=600\;\mathrm{cal}
\end{gather}
\]
\[
\begin{gather}
E=Q=600\;\mathrm{cal}
\end{gather}
\]
The equation of heat is given by
\[
\begin{gather}
\bbox[#99CCFF,10px]
{Q=mc\Delta\theta}
\end{gather}
\]
\[
\begin{gather}
\Delta \theta=\frac{Q}{mc} \\[5pt]
\Delta \theta=\frac{600}{100\times 1}
\end{gather}
\]
\[
\begin{gather}
\bbox[#FFCCCC,10px]
{\Delta \theta =6\;\mathrm{°C}}
\end{gather}
\]
Note: the symbol θ was used for the temperature, so as not to confuse it with
t used for the time interval in the power formula.
advertisement

Fisicaexe - Physics Solved Problems by Elcio Brandani Mondadori is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License .