Solved Problem on Heat
advertisement
Determine the heat required to convert 100 g of ice at −10 °C in 100 g of steam at 100 °C. Also, plot a temperature graph as a function of the quantity of heat of the phase changes. Data:
specific heat of ice: ci = 0.5 cal/g°C;
latent heat of fusion: LF = 80 cal/g;
specific heat of water: cw = 1.0 cal/g°C;
latent heat of vaporization: Lv = 540 cal/g.
Problem data:
- Mass of ice: m = 100 g;
- Initial temperature of ice: ti = −10 °C;
- Final temperature of steam: tf = 100 °C;
- Specific heat of ice: ci = 0.5 cal/g°C;
- Latent heat of fusion: LF = 80 cal/g;
- Specific heat of water: cw = 1.0 cal/g°C;
- Latent heat of vaporization: Lv = 540 cal/g.
- 1.ª Part
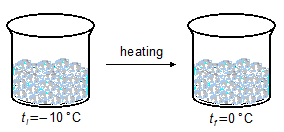
\[
\begin{gather}
\bbox[#99CCFF,10px]
{Q=mc\Delta t} \tag{I}
\end{gather}
\]
\[
\begin{gather}
Q_1=mc_i\Delta t \\[5pt]
Q_1=m c_i(t_0-t_{-10}) \\[5pt]
Q_1=(100\;\mathrm{\cancel g})\left(0.5\;\mathrm{\small{\frac{cal}{\cancel g\cancel{°C}}}}\right)[0-(-10)]\;\mathrm{\cancel{°C}} \\[5pt]
Q_1=100\times(0.5\;\mathrm{cal})\times 10 \\[5pt]
Q_1=500\;\mathrm{cal}
\end{gather}
\]
- 2.ª Part
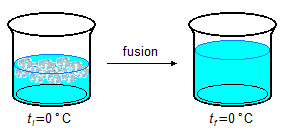
\[
\begin{gather}
\bbox[#99CCFF,10px]
{Q=mL} \tag{I}
\end{gather}
\]
\[
\begin{gather}
Q_2=mL_F \\[5pt]
Q_2=100\;\mathrm{\cancel g}\times 80\;\mathrm{\small{\frac{cal}{\cancel g}}} \\[5pt]
Q_2=8000\;\mathrm{cal}
\end{gather}
\]
- 3.ª Part
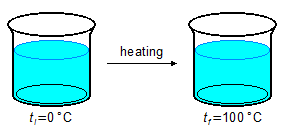
\[
\begin{gather}
Q_3=mc_w\Delta t \\[5pt]
Q_3=m c_w(t_{100}-t_0) \\[5pt]
Q_3=(100\;\mathrm{\cancel g})\left(1.0\;\mathrm{\small{\frac{cal}{\cancel g\cancel{°C}}}}\right)(100-0)\;\mathrm{\cancel{°C}} \\[5pt]
Q_3=100\times(1.0\;\mathrm{cal})\times 100 \\[5pt]
Q_3=10000\;\mathrm{cal}
\end{gather}
\]
- 4.ª Part
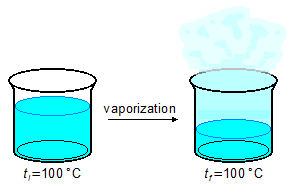
\[
\begin{gather}
Q_4=mL_v \\[5pt]
Q_4=100\;\mathrm{\cancel g}\times 540\;\mathrm{\small{\frac{cal}{\cancel g}}} \\[5pt]
Q_4=54000\;\mathrm{cal}
\end{gather}
\]
Thus the total heat to convert 100 g of ice into −10 °C of steam at 100 °C will be the sum of all
parts calculated above.
\[
\begin{gather}
Q=Q_1+Q_2+Q_3+Q_4 \\[5pt]
Q=(500+8000+10000+54000)\;\mathrm{cal}
\end{gather}
\]
\[
\begin{gather}
\bbox[#FFCCCC,10px]
{Q=72500\;\mathrm{cal}}
\end{gather}
\]
Plotting in a graph the values of the temperatures of each phase of the transformations and the amounts
of heat accumulated at each phase, we have the graph of Figure 5 below
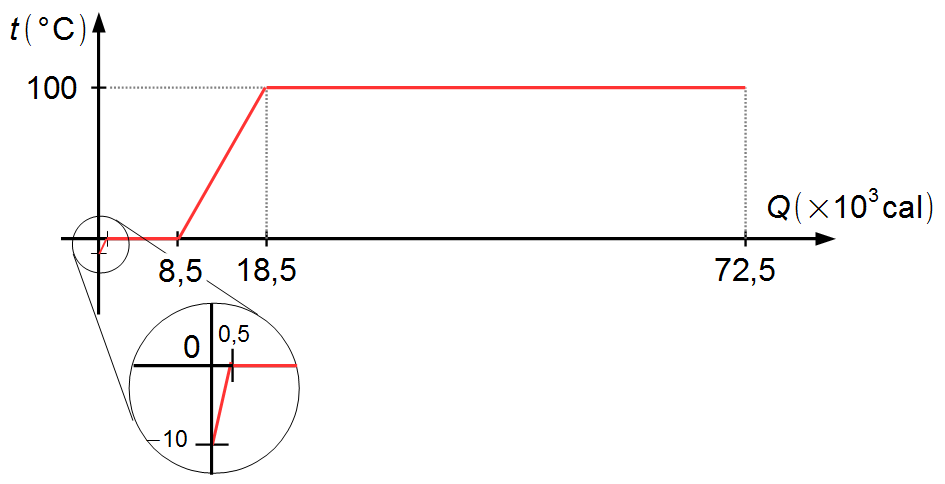
Note: if the graph is plotted to scale, the amount of heat required to heat the ice from
−10 °C to 0 °C is represented by a very small section (highlighted), while the amount of heat
needed to vaporize the water at 100 °C occupies most of the graph.
advertisement

Fisicaexe - Physics Solved Problems by Elcio Brandani Mondadori is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License .