Solved Problem on Gases
advertisement
A mercury barometer has a small amount of air at the top of the tube, so its readings are incorrect. For
an actual pressure reading of 756 mm of mercury, it reads 748 mm, and for an actual reading of 738 mm,
it reads 734 mm.
a) What is the length L of the tube of this barometer?
b) For a reading of 745 mm of mercury, what is the actual atmospheric pressure?
a) What is the length L of the tube of this barometer?
b) For a reading of 745 mm of mercury, what is the actual atmospheric pressure?
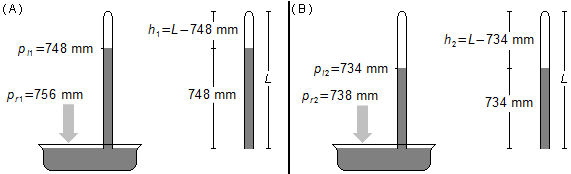
Problem data:
- Actual pressure in situation 1: pr1 = 756 mm Hg;
- Pressure indicated in situation 1: pi1 = 748 mm Hg;
- Actual pressure in situation 2: pr2 = 738 mm Hg;
- Pressure indicated in situation 2: pi2 = 734 mm Hg.
Solution
a) Initially (Figure 1-A), the real pressure of the place is 756 mm, the value indicated by the mercury column of the barometer is 748 mm, as L is the total length of the tube, the height of the column of air trapped in the top of the barometer is
\[
\begin{gather}
h_{1}=L-p_{i1} \tag{I}
\end{gather}
\]
The atmospheric pressure, pr1, is balanced by the pressure of the mercury column,
pi1, and the air inside the tube, p1
\[
\begin{gather}
p_{r1}=p_{i1}+p_{1}\\[5pt]
p_{1}=p_{r1}-p_{i1} \tag{II}
\end{gather}
\]
In the final situation (Figure 1-B) the actual pressure is 738 mm and the barometer reading is 734 mm, the
height of the air column will be
\[
\begin{gather}
h_{2}=L-p_{i2} \tag{III}
\end{gather}
\]
Again the atmospheric pressure is balanced by the pressure of the mercury column and the air inside the tube.
\[
\begin{gather}
p_{r2}=p_{i2}+p_{2}\\[5pt]
p_{2}=p_{r2}-p_{i2} \tag{IV}
\end{gather}
\]
Applying the General Gas Equation
\[
\begin{gather}
\bbox[#99CCFF,10px]
{\frac{p_{1}V_{1}}{T_{1}}=\frac{p_{2}V_{2}}{T_{2}}}
\end{gather}
\]
as the initial and final temperatures are the same, T1 = T2, we have an
isothermal transformation given by Boyle's Law
\[
\begin{gather}
\bbox[#99CCFF,10px]
{p_{1}V_{1}=p_{2}V_{2}} \tag{V}
\end{gather}
\]
Since A is the cross-sectional area of the barometer tube, the volumes of the air columns at the top
of the tube in both situations are given by
\[
\begin{gather}
\bbox[#99CCFF,10px]
{V=Ah}
\end{gather}
\]
\[
\begin{gather}
V_{1}=Ah_{1} \tag{VI-a}
\end{gather}
\]
\[
\begin{gather}
V_{2}=Ah_{2} \tag{VI-b}
\end{gather}
\]
substituting the values of expressions (VI-a) and (VI-b) into expression (V)
\[
\begin{gather}
p_{1}\cancel{A}h_{1}=p_{2}\cancel{A}h_{2} \tag{VII}
\end{gather}
\]
substituting expressions (I), (II), (III), and (IV) into expression (VII)
\[
\begin{gather}
(p_{r1}-p_{i1})(L-p_{i1})=(p_{r2}-p_{i2})(L-p_{i2})\\[5pt]
(p_{r1}-p_{i1})L-(p_{r1}-p_{i1})p_{i1}=(p_{r2}-p_{i2})L-(p_{r2}-p_{i2})p_{i2}\\[5pt]
(p_{r1}-p_{i1})L-(p_{r2}-p_{i2})L=(p_{r1}-p_{i1})p_{i1}-(p_{r2}-p_{i2})p_{i2}\\[5pt]
L[(p_{r1}-p_{i1})-(p_{r2}-p_{i2})]=(p_{r1}-p_{i1})p_{i1}-(p_{r2}-p_{i2})p_{i2}\\[5pt]
L=\frac{(p_{r1}-p_{i1})p_{i1}-(p_{r2}-p_{i2})p_{i2}}{(p_{r1}-p_{i1})-(p_{r2}-p_{i2})} \tag{VIII}
\end{gather}
\]
substituting the values of the problem, we get the length of the tube
\[
\begin{gather}
L=\frac{(756-748).748-(738-734).734}{(756-748)-(738-734)}\\[5pt]
L=\frac{8\times 748-4\times 734}{8-4}\\[5pt]
L=\frac{8\times 748-4\times 734}{4}\\[5pt]
L=\frac{\cancel{4}(2\times 748-734)}{\cancel{4}}\\[5pt]
L=1496-734
\end{gather}
\]
\[
\begin{gather}
\bbox[#FFCCCC,10px]
{L=762\;\text{mm}}
\end{gather}
\]
b) For an indicated pressure of pL = 745 mm, and using the value found in the previous item, the actual atmospheric pressure can be found using expression (VIII). Substituting the values of the situation shown in Figure 1-A and pi2 = pi and pr2 = pr
\[
\begin{gather}
762=\frac{(756-748)\times 748-(p_{r}-745)\times 745}{(756-748)-(p_{r}-745)}\\[5pt]
762=\frac{8\times 748-(p_{r}-745)\times 745}{8-(p_{r}-745)}\\[5pt]
762\times [8-(p_{r}-745)]=8\times 748-(p_{r}-745)\times 745\\[5pt]
8\times 762-(p_{r}-745)\times 762=8\times 748-(p_{r}-745)\times 745\\[5pt]
(p_{r}-745)\times 762-(p_{r}-745)\times 745=8\times 762-8\times 748\\[5pt]
(p_{r}-745)\times(762-745)=8\times(762-748)\\[5pt]
(p_{r}-745)\times17=8\times14\\[5pt]
p_{r}-745=\frac{8\times 14}{17}\\[5pt]
p_{r}=745+6.6
\end{gather}
\]
\[
\begin{gather}
\bbox[#FFCCCC,10px]
{p_{r}=751.6\;\text{mm Hg}}
\end{gather}
\]
Note: In the same way, if we substitute the values of the situation shown in Figure 1-B,
with pi1 = pi and pr1 =
pr
\[
\begin{gather}
762=\frac{(p_{r}-745)\times 745-(738-734)\times 734}{(p_{r}-745)-(738-734)}\\[5pt]
762=\frac{(p_{r}-745)\times 745-4\times 734}{(p_{r}-745)-4}\\[5pt]
762.[(p_{r}-745)-4]=(p_{r}-745)\times 745-4\times 734\\[5pt]
(p_{r}-745)\times 762-4\times 762=(p_{r}-745)\times 745-4\times 734\\[5pt]
(p_{r}-745)\times 762-(p_{r}-745)\times 745=4\times 762-4\times 734\\[5pt]
(p_{r}-745)\times(762-745)=4\times(762-734)\\[5pt]
(p_{r}-745)\times 17=4\times 28\\[5pt]
p_{r}-745=\frac{4\times 28}{17}\\[5pt]
p_{r}=745+6.6\\[5pt]
p_{r}=751.6\;\text{mm Hg}
\end{gather}
\]
and we get the same result as in (b).
advertisement

Fisicaexe - Physics Solved Problems by Elcio Brandani Mondadori is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License .