Solved Problem on Gases
advertisement
A glass capillary closed at the lower end and open at the upper end has air trapped at the bottom by a
column of mercury as shown in the figure. The capillary is inclined at 60° from the vertical. What is
the length of the air column in this condition? Given: local air pressure 1.0×105 Pa,
the density of mercury 13.6 g/cm3, and acceleration due to gravity is 9.8 m/s2.
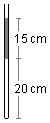
Problem data:
- Height of the column of mercury: h = 15 cm;
- Initial height of the air column: h1 = 20 cm;
- Capillary inclination: 60°;
- Atmospheric pressure: p0 = 1.0×105 Pa;
- Density of mercury: μ = 13.6 g/cm3;
- Acceleration due to gravity: g = 9.8 m/s2.
In the initial situation (Figure 1-A), the column of air is under the pressure of the column of mercury and atmospheric pressure. When the capillary is inclined, we have only the vertical component, H, which contributes to exerting pressure on the air column (Figure 1-B).
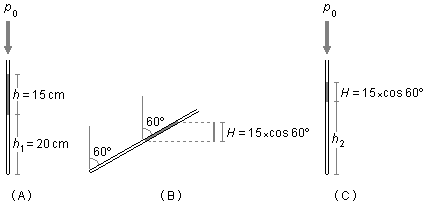
This situation is equivalent to the vertical capillary with the air column trapped by a mercury column of height H and atmospheric pressure (Figure 1-C).
Solution
First, we must convert the density of mercury given in grams per cubic centimeter (g/cm3) to kilograms per cubic meter (kg/m3) and the capillary measurements given in centimeters (cm) to meters (m) used in the International System of Units (SI)
\[
\begin{gather}
\mu=13.6\;\frac{\cancel{\text{g}}}{\text{cm}^{3}}\times\frac{10^{-3}\;\text{kg}}{1\;\cancel{\text{g}}}\times\frac{(1\;\text{cm})^{3}}{(10^{-2}\;\text{m})^{3}}=13.6\;\frac{1}{\cancel{\text{cm}^{3}}}\;\text{kg}\times\frac{1\;\cancel{\text{cm}^{3}}}{10^{-6}\;\text{m}^{3}}=13.6\times 10^{-3}\times 10^{6}\;\frac{\text{kg}}{\;\text{m}^{3}}=13.6\times 10^{3}\;\frac{\text{kg}}{\;\text{m}^{3}}\\[10pt]
h=15\;\text{cm}=15\times 10^{-2}\;\text{m}=0.15\;\text{m}\\[10pt]
h_{1}=20\;\text{cm}=20\times 10^{-2}\;\text{m}=0.20\;\text{m}
\end{gather}
\]
Using the General Gas Equation
\[
\begin{gather}
\bbox[#99CCFF,10px]
{\frac{p_{1}V_{1}}{T_{1}}=\frac{p_{2}V_{2}}{T_{2}}}
\end{gather}
\]
as the initial and final temperatures are the same, T1 = T2, we have an
isothermal process given by Boyle's Law
\[
\begin{gather}
\bbox[#99CCFF,10px]
{p_{1}V_{1}=p_{2}V_{2}} \tag{I}
\end{gather}
\]
The pressure is given by Stevin's Law
\[
\begin{gather}
\bbox[#99CCFF,10px]
{p=p_{0}+\mu gh} \tag{II}
\end{gather}
\]
since the capillary is a cylinder, the volume is given by
\[
\begin{gather}
\bbox[#99CCFF,10px]
{V=Ah} \tag{III}
\end{gather}
\]
Applying expression (II) to the initial situation
\[
\begin{gather}
p_{1}=p_{0}+\mu gh \tag{IV}
\end{gather}
\]
applying expression (III), we have the volume of the capillary
\[
\begin{gather}
V_{1}=Ah_{1} \tag{V}
\end{gather}
\]
After inclining the capillary, the pressure on the air column (air pressure plus the pressure of the
vertical component of the mercury column) will be given by applying expression (II)
\[
\begin{gather}
p_{2}=p_{0}+\mu gH
\end{gather}
\]
where the height H is (Figure 1-B)
\[
\begin{gather}
\cos 60°=\frac{\text{cateto adjacente}}{\text{hipotenusa}}=\frac{H}{h}\\H=h\cos 60°
\end{gather}
\]
therefore
\[
\begin{gather}
p_{2}=p_{0}+\mu gh\cos 60° \tag{VI}
\end{gather}
\]
and for the volume of the air column
\[
\begin{gather}
V_{2}=Ah_{2} \tag{VII}
\end{gather}
\]
Substituting expressions (IV), (V), (VI), and (VII) into expression (I)
\[
\begin{gather}
(p_{0}+\mu gh)Ah_{1}=(p_{0}+\mu gh\cos 60°)Ah_{2}\\[5pt]
(p_{0}+\mu gh)h_{1}=(p_{0}+\mu gh\cos 60°)h_{2}\\[5pt]
h_{2}=\frac{(p_{0}+\mu gh)h_{1}}{p_{0}+\mu gh\cos 60°}
\end{gather}
\]
substituting the numerical data of the problem
\( \cos 60°=\dfrac{1}{2} \)
\[
\begin{gather}
h_{2}=\frac{(1.0\times 10^{5}+13.6\times 10^{3}\times 9.8\times 0.15)\times 0.20}{1.0\times 10^{5}+13.6\times 10^{3}\times 9.8\times 0.15.\dfrac{1}{2}}\\[5pt]
h_{2}=0.22\;\text{m}
\end{gather}
\]
\[
\begin{gather}
\bbox[#FFCCCC,10px]
{h_{2}=22\;\text{cm}}
\end{gather}
\]
advertisement

Fisicaexe - Physics Solved Problems by Elcio Brandani Mondadori is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License .