Solved Problem on Quantum Physics
advertisement
A cesium photoelectron has a kinetic energy of 2 eV.
a) What is the maximum frequency of light that could have emitted this electron? Data: Cesium work function = 1.8 eV, Planck's constant h = 6.62×10−34 J.s, 1 eV = 1.6×10−19 J.
b) What is the maximum wavelength of this frequency? Data: speed of light c = 3×108 m/s.
Problem data:
- Cesium photoelectron kinetic energy: Ec = 2 eV;
- Cesium work function: φ = 1.8 eV;
- Planck's constant: h = 6.62 ×10−34 J.s;
- Conversion factor of electron-volt/Joule: 1 eV = 1.6 ×10−19 J;
- Speed of light: c = 3 ×108 m/s.
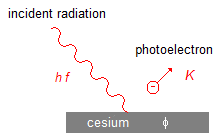
Radiation of frequency f and energy hf incident on a cesium surface that emits an electron
with energy K (Figure 1).
Solution
First, we must convert the kinetic energy and work function values given in electron volts (eV) to joules (J), to make it compatible with the value of Planck's Constant h given in joules.second (J.s).
For kinetic energy using a cross-multiplication
\[
\begin{gather}
\frac{1\;\text{eV}}{1.6\times 10^{-19}\;\text{J}}=\frac{2\;\text{eV}}{K}\\[5pt]
K=\frac{2\;\cancel{\text{eV}}\times 1.6\times 10^{-19}\;\text{J}}{1\;\cancel{\text{eV}}}\\[5pt]
K=3.2\times 10^{-19}\;\text{J}
\end{gather}
\]
For the work function
\[
\begin{gather}
\frac{1\;\text{eV}}{1.6\times 10^{-19}\;\text{J}}=\frac{1.8\;\text{eV}}{\phi}\\[5pt]
\phi=\frac{1.8\;\cancel{\text{eV}}\times 1.6\times 10^{-19}\;\text{J}}{1\;\cancel{\text{eV}}}\\[5pt]
\phi=2.88\times 10^{-19}\;\text{J}
\end{gather}
\]
a) For the given kinetic energy the maximum frequency will be given by
\[
\begin{gather}
\bbox[#99CCFF,10px]
{hf=\phi +E_{c}}
\end{gather}
\]
\[
\begin{gather}
6.62\times 10^{-34}f=(2.88-3.2)\times 10^{-19}\\[5pt]
f=\frac{6,08.10^{-19}}{6.62\times 10^{-34}}
\end{gather}
\]
\[
\begin{gather}
\bbox[#FFCCCC,10px]
{f=9.2\times 10^{14}\;\text{Hz}}
\end{gather}
\]
b) Frequency and wavelength are related by
\[
\begin{gather}
\bbox[#99CCFF,10px]
{c=\lambda f}
\end{gather}
\]
\[
\begin{gather}
\lambda =\frac{c}{f}
\end{gather}
\]
substituting the speed of light c given in the statement and the frequency f found in the
previous item
\[
\begin{gather}
\lambda =\frac{3\times 10^{8}}{9.2\times 10^{14}}
\end{gather}
\]
\[
\begin{gather}
\bbox[#FFCCCC,10px]
{\lambda =3.3\times10^{7}\;\text{m}}
\end{gather}
\]
advertisement

Fisicaexe - Physics Solved Problems by Elcio Brandani Mondadori is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License .