Solved Problem on Thermodynamics
advertisement
A gas undergoes a process shown in graph p=f(T). Knowing the
Universal Gas Constant R=8.31 J/mol.K, the number of moles of the gas n=5, the
molar specific heat of the constant volume CV=5 cal/mol.k and 1 cal = 4. 18 J.
Determine:
a) The type of thermodynamic process undergone by the gas;
b) The volume of gas during the process;
c) the amount of heat that the gas receives during the process;
d) The change of the internal energy of the gas in this process.
a) The type of thermodynamic process undergone by the gas;
b) The volume of gas during the process;
c) the amount of heat that the gas receives during the process;
d) The change of the internal energy of the gas in this process.
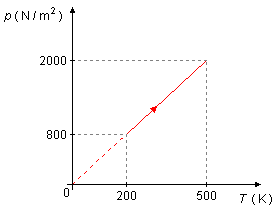
Problem data:
- Number of moles of gas: n = 5 mol;
- Molar specific heat of the constant volume: CV = 5 cal/mol.K;
- Universal Gas Constant: R = 8.31 J/mol.K;
- Mechanical equivalent of heat: 1 cal = 4.18 J.
a) In the thermodynamic process, the combined gas law gives \( \dfrac{pV}{T}=k \), where k is constant, so \( p=\dfrac{k}{V}T \), the graph shows to us that the process is linear, so \( \dfrac{k}{V} \) is constant, so V is also constant, and the process is isovolumetric (or isometric or isochoric).
Note: The expression
\( p=\frac{k}{V}T \)
that characterizes the isovolumetric process is a linear function of type
\( y=ax+b \),
where we can do the following associations
\[
\begin{array}{c}
y & = & a & x & + & b \\
{\color{red}\downarrow} & & {\color{red}\downarrow} & {\color{red}\downarrow} & & {\color{red}\downarrow} \\
p & = & \dfrac{k}{V} & T & + & 0
\end{array}
\]
b) From the graph we get the pair of values T = 500 k and p = 2000 N/m2, using the Ideal Gas Law
\[ \bbox[#99CCFF,10px]
{pV=nRT}
\]
and the problem data
\[
\begin{gather}
2000V=5\times 8.31\times 500\\
V=\frac{20775}{2000}
\end{gather}
\]
\[ \bbox[#FFCCCC,10px]
{V=10.4\;\text{m}^{3}}
\]
c) The amount of heat received by the gas is given by
\[ \bbox[#99CCFF,10px]
{Q=nC_{V}\Delta T}
\]
The initial temperature of the gas is T1 = 200 K, and the final temperature of
T2 = 500 K, the change in temperature will be
\( \Delta T=T_{2}-T_{1}=500-200=300\;\text{K} \)
\( \Delta T=T_{2}-T_{1}=500-200=300\;\text{K} \)
and using the other problem data
\[
\begin{gather}
Q=5\times 5\times 300\\
Q=7500\;\text{cal}
\end{gather}
\]
converting this value to joules
\[
Q=7500\;\cancel{\text{cal}}\times\frac{4,18\;\text{J}}{1\;\cancel{\text{cal}}}
\]
\[ \bbox[#FFCCCC,10px]
{Q=31350\;\text{J}}
\]
d) The First Law of Thermodynamics is given by
\[ \bbox[#99CCFF,10px]
{\Delta U=Q-W}
\]
and the work W is given by
\[ \bbox[#99CCFF,10px]
{W=p\Delta V}
\]
but the process is isovolumetric, so ΔV is equal to zero, and we have to ΔU = Q
\[ \bbox[#FFCCCC,10px]
{\Delta U=31350\;\text{J}}
\]
advertisement

Fisicaexe - Physics Solved Problems by Elcio Brandani Mondadori is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License .