Solved Problem on Gases
advertisement
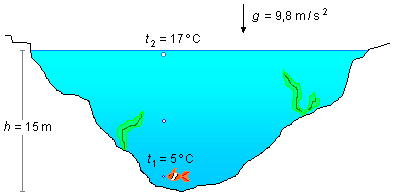
A fish at the bottom of a lake 15 m deep emits an air bubble of volume V0, the temperature at the bottom of the lake being 5 °C and at the surface 17 °C. Calculate the volume of the bubble when it reaches the surface of the lake. Data: atmospheric pressure p0 = 1.01×105 Pa, water density μ = 1.0 g/cm3, and acceleration due to gravity g = 9.8 m/s2.
Problem data:
- Initial volume of bubble: V0;
- Lake depth: h = 15 m;
- Temperature at the bottom of the lake: t1 = 5 °C;
- Temperature at the surface of the lake: t2 = 17 °C;
- Atmospheric pressure: p0 = 1.01×105 Pa;
- Density of water: μ = 1.0 g/cm3;
- Acceleration due to gravity: g = 9.8 m/s2.
Solution
First, we must convert the density of water given in grams per cubic centimeter (g/cm3) to kilograms per cubic meter (kg/m3) used in the International System of Units (SI) and temperatures from degrees Celsius (°C) to Kelvins ( K).
\[
\begin{gather}
\mu=1.0\;\frac{\cancel{\text{g}}}{\text{cm}^{3}}\times\frac{10^{-3}\;\text{kg}}{1\;\cancel{\text{g}}}\times\frac{(1\;\text{cm})^{3}}{(10^{-2}\;\text{m})^{3}}=1.0\;\frac{1}{\cancel{\text{cm}^{3}}}\;\text{kg}\times\frac{1\;\cancel{\text{cm}^{3}}}{10^{-6}\;\text{m}^{3}}=1.0\times 10^{-3}\times 10^{6}\;\frac{\text{kg}}{\;\text{m}^{3}}=1.0\times 10^{3}\;\frac{\text{kg}}{\;\text{m}^{3}}\\[10pt]
T_{1}=t_{1C}+273=5+273=278\;\text{K}\\[10pt]
T_{2}=t_{2C}+273=17+273=290\;\text{K}
\end{gather}
\]
At the bottom of the lake, the air bubble is under the pressure of the water column above it and the
atmospheric pressure above the lake (Figure 2), using Stevin's Law
\[
\begin{gather}
\bbox[#99CCFF,10px]
{p_{1}=p_{0}+\mu gh} \tag{I}
\end{gather}
\]
the volume will be
\[
\begin{gather}
V_{1} = V_{0} \tag{II}
\end{gather}
\]

When the bubble reaches the surface it is only under the action of atmospheric pressure (Figure 3)
\[
\begin{gather}
p_{2} = p_{0} \tag{III}
\end{gather}
\]

Considering the air bubble to be a perfect gas, we use the General Gas Equation applied to situations at the bottom and surface of the lake.
\[
\begin{gather}
\bbox[#99CCFF,10px]
{\frac{p_{1}V_{1}}{T_{1}}=\frac{p_{2}V_{2}}{T_{2}}} \tag{IV}
\end{gather}
\]
substituting the values of (I), (II), and (III) into the expression (IV)
\[
\begin{gather}
\frac{(p_{0}+\mu
gh)V_{0}}{T_{1}}=\frac{p_{0}V_{2}}{T_{2}}\\[5pt]
V_{2}=\frac{T_{2}}{T_{1}}\frac{(p_{0}+\mu
gh)}{p_{0}}V_{0}
\end{gather}
\]
substituting the values
\[
\begin{gather}
V_{2}=\frac{T_{2}}{T_{1}}\frac{(p_{0}+\mu gh)}{p_{0}}V_{0}\\[5pt]
V_{2}=\frac{290}{278}\times \frac{(1.01\times 10^{5}+1.0\times 10^{3}\times 9.8\times 15)}{1.01\times 10^{5}}V_{0}\\[5pt]
V_{2}=1.04\times \frac{(1.01\times 10^{5}+1.47\times 10^{5})}{1.01\times 10^{5}}V_{0}\\[5pt]
V_{2}=1.04\times \frac{2.48\times 10^{5}}{1.01\times 10^{5}}V_{0}
\end{gather}
\]
\[
\begin{gather}
\bbox[#FFCCCC,10px]
{V_{2}=2.6V_{0}}
\end{gather}
\]
advertisement

Fisicaexe - Physics Solved Problems by Elcio Brandani Mondadori is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License .