Solved Problem on Temperature
advertisement
In a constant-pressure gas thermometer, the physical quantity is the gas volume. The calibration
of the thermometer is given by the graph shown in the figure. Determine:
a) The equation of that thermometer;
b) When the volume of the gas is 130 cm3, what will be the temperature of the gas?
a) The equation of that thermometer;
b) When the volume of the gas is 130 cm3, what will be the temperature of the gas?
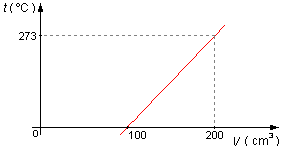
Problem data:
- For volume:
- First calibration point: V1 = 100 cm3;
- Second calibration point: V2 = 200 cm3;
- For temperature:
- First calibration point: t1 = 0 °C;
- Second calibration point: t2 = 273 °C.
We chose any point (V, t) of the graph, two other points are
(V1, t1)=(100, 0) and
(V2, t2)=(200, 273).
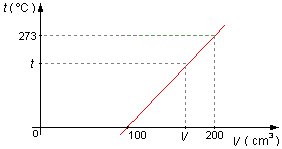
Solution
a) To find the equation of this thermometer, we have
\[ \bbox[#99CCFF,10px]
{\frac{g-g_{1}}{g_{2}-g_{1}}=\frac{t-t_{1}}{t_{2}-t_{1}}}
\]
where physical quantity g for this problem is the volume of the gas V
\[
\frac{V-V_{1}}{V_{2}-V_{1}}=\frac{t-t_{1}}{t_{2}-t_{1}}
\]
substituting the problem data, we have
\[
\begin{gather}
\frac{V-100}{200-100}=\frac{t-0}{273-0}\\
\frac{V-100}{100}=\frac{t}{273}\\
273\times (V-100)=100t\\
273V-273\times 100=100t\\
100t=273V-27300\\
t=\frac{273}{100}V-\frac{27300}{100}
\end{gather}
\]
the equation that gives us the temperature as a function of volume
t = f(V), will be
\[ \bbox[#FFCCCC,10px]
{t=2.73V-273}
\]
b) For V = 130 cm3, we have substituted this value in the equation found in the previous item to obtain
\[
t=2.73\times 130-273
\]
\[ \bbox[#FFCCCC,10px]
{t=81.9 °\text{C}}
\]
advertisement

Fisicaexe - Physics Solved Problems by Elcio Brandani Mondadori is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License .